
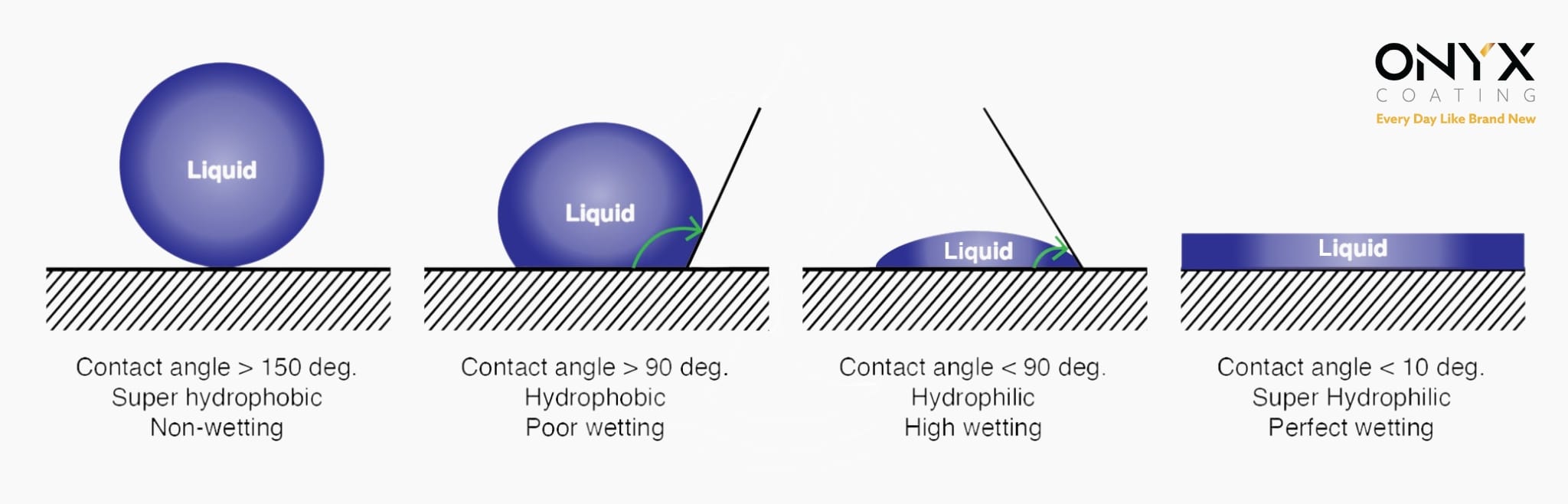
Hydrophobicity refers to the property of “repelling or not mixing with water.” Coatings that create a hydrophobic or superhydrophobic surface provide several benefits to the coated surface and the underlying material. These benefits include reduced dirt accumulation, self-cleaning properties, enhanced resistance to moisture and corrosion, and increased durability of both the coating and the substrate. Understanding and measuring hydrophobicity requires examining the relationship between contact angle and the hydrophobic or hydrophilic nature of a surface.
The surface characteristics of a material can give rise to various types of coatings, ranging from hydrophilic (water-attracting) to superhydrophobic (highly water-repellent) coatings. The contact angle of a water droplet on the surface of a coating is influenced by several factors, including the macro, micro, and nanoscale surface profiles, as well as the surface tension of the coating material. Surface tension refers to the elastic property of liquids that causes them to minimize their surface area.
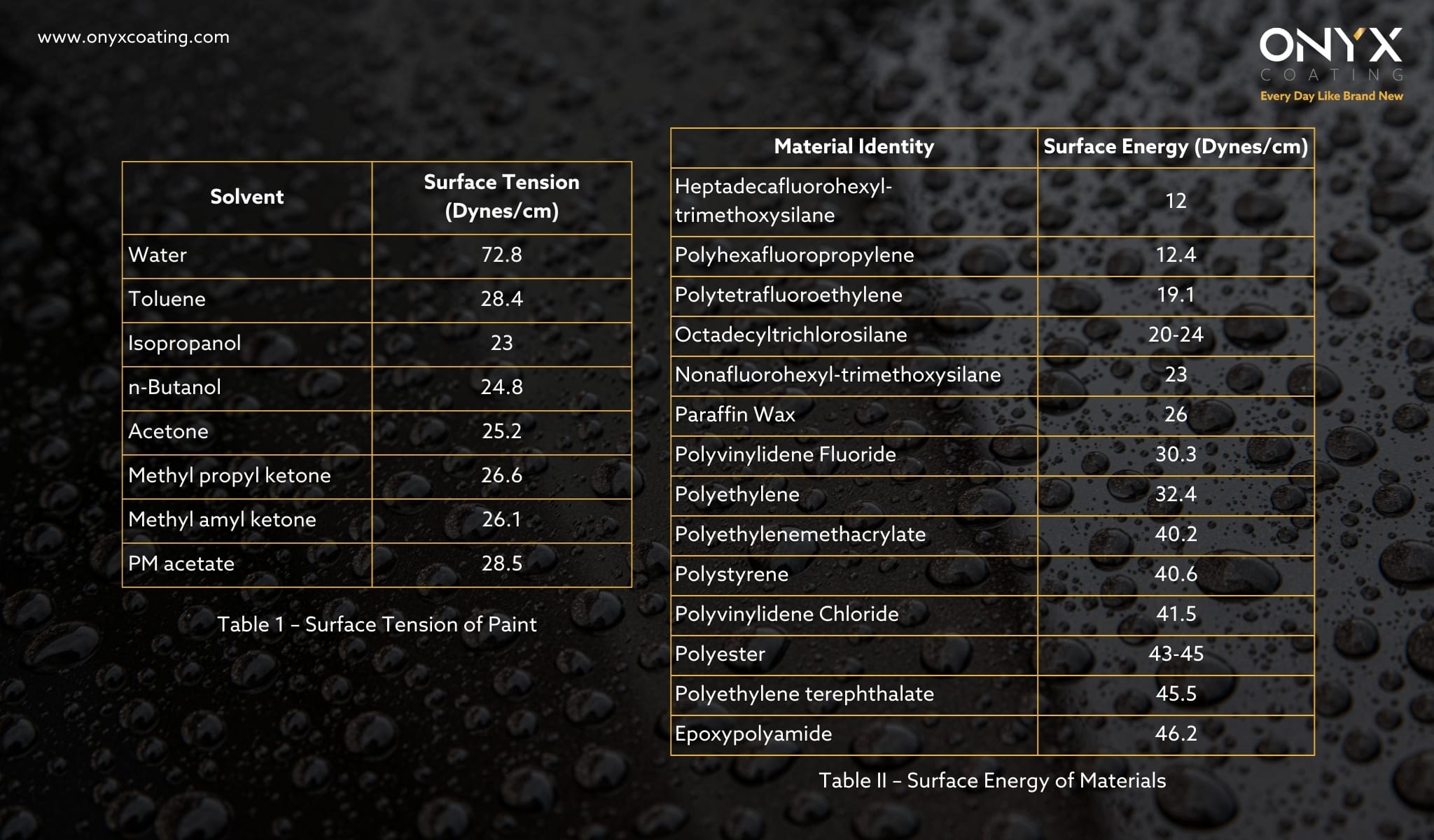
As shown in table 1, water has a higher surface tension compared to many common solvents used in the paint industry. This is primarily due to the strong hydrogen bonding between water molecules. Additionally, the microscopic surface geometry plays a critical role in determining the hydrophobic nature of a coating.
Nature provides numerous examples of hydrophobic and superhydrophobic surfaces, with one of the most remarkable being the Lotus leaf. Water on a Lotus leaf exhibits a contact angle exceeding 150°, a key indicator of its extreme water-repellent properties. The self-cleaning ability of the Lotus leaf stems from its hydrophobic, double-layered surface structure. This unique design minimizes the contact area and adhesion forces between the water droplets and the leaf surface, allowing droplets to easily roll off, collecting dust and debris in the process. The micron-scale double structure of the leaf consists of needle-like projections covered by a waxy layer, enhancing its water-repellent and self-cleaning capabilities.
The wax-covered projections on surfaces like the Lotus leaf are approximately 10 to 20 µm in height and 10 to 15 µm in width. These waxes, which form the upper layer of the leaf’s double structure, are inherently hydrophobic. Some plants exhibit contact angles as high as 160°, classifying them as superhydrophobic. In such cases, only 2–3% of a water droplet’s surface area comes into contact with the plant surface. When the contact area is reduced to less than 0.6%, the self-cleaning effect becomes highly pronounced.
To this point, we’ve explored the factors influencing hydrophobicity, including contact angle, surface structure, and the wetting behavior of organic solvents compared to water, which is driven by their lower surface tension. The next step focuses on strategies to enhance the hydrophobicity of coating systems, particularly from a surface engineering perspective.
To optimize the hydrophobicity of a coating, it is essential to minimize the surface energy of the coating material. Lower surface energy, combined with a well-structured surface, significantly enhances hydrophobic performance. Surface energy is measured in the same units as surface tension (force per unit length, such as dynes/cm). Liquids with high surface tension, like water, exhibit poor wetting (high contact angle) on coatings with low surface energy, leading to maximum hydrophobicity. As shown in Table II, the surface energy of materials varies considerably based on their composition and interaction with water.
For instance, a coating made with polyhexafluoropropylene, which has a surface energy of 12.0 dynes/cm, will produce a more hydrophobic surface than one made with polymethylmethacrylate, which has a surface energy of 40.2 dynes/cm. To maximize hydrophobicity, the most hydrophobic functional groups of the material should be oriented at the surface.
For example, if an organofunctional trimethoxysilane is utilized for surface modification, its methoxysilane groups should be engineered to occupy the surface layer. Similarly, perfluoro and aliphatic groups positioned at the coating surface provide significantly higher hydrophobicity compared to ester or alcohol groups. The hierarchy of surface tension, from lowest to highest, emphasizes the importance of careful material selection and surface engineering in achieving superior hydrophobic performance.
Providing increased hydrophobicity throughout a properly engineered coating can also provide additional attributes such as improved corrosion and moisture resistance.
For more information visit Prospector® Knowledge Center
ONYX COATING‘s Hydrophobic Coatings offer cutting-edge water-repellent solutions designed to protect surfaces from moisture, dirt, and corrosion. With advanced formulations that reduce surface friction and enhance self-cleaning properties, ONYX COATING’s hydrophobic coatings ensure longer-lasting protection for vehicles, electronics, and various industrial applications. By minimizing water adhesion, these coatings not only improve surface durability but also reduce maintenance efforts, keeping your assets looking pristine and functioning optimally.

Watch as water beads off the deck with our super hydrophobic coatings.

Protect the paintwork from UV damage and keep its vibrant colour despite the conditions.

Let the fear of a rough docking be a thing of the past with a 10H coating.

With minimal maintenance, our products are guaranteed to provide protection for years to come.

With our chemical and contaminant resistant coating, the surface is kept clean. Needing only minor occasional maintenance.

Trusted in 70+ countries, Onyx Coating is the top choice for professionals and car owners seeking premium protection.

Our ceramic coatings adhere to the surface making it resistant to corrosion and oxidation and contamination-free.

Bonds tight with the surtace to maintain a uniform cover and protect the surface evenly.
If you are interested in our products or keen to work with us, send us a message and we will get in contact with you to schedule a quick call or contact us via our email [email protected]
Once this information has been received our team will review the accounts and get into contact with you.
With the world’s first N1 nano coating product under our name, we are one of the most advanced automotive protection providers in the market.
Sign up for the latest offers & promotions




© ONYX COATING 2025. All Rights Reserved.